Purificationofthecreatinekinase.docx
《Purificationofthecreatinekinase.docx》由会员分享,可在线阅读,更多相关《Purificationofthecreatinekinase.docx(16页珍藏版)》请在冰点文库上搜索。
Purificationofthecreatinekinase
Isolationandenzymaticanalysisofcreatinekinase
ofrabbitmuscle
●Background
1Creatinkinase:
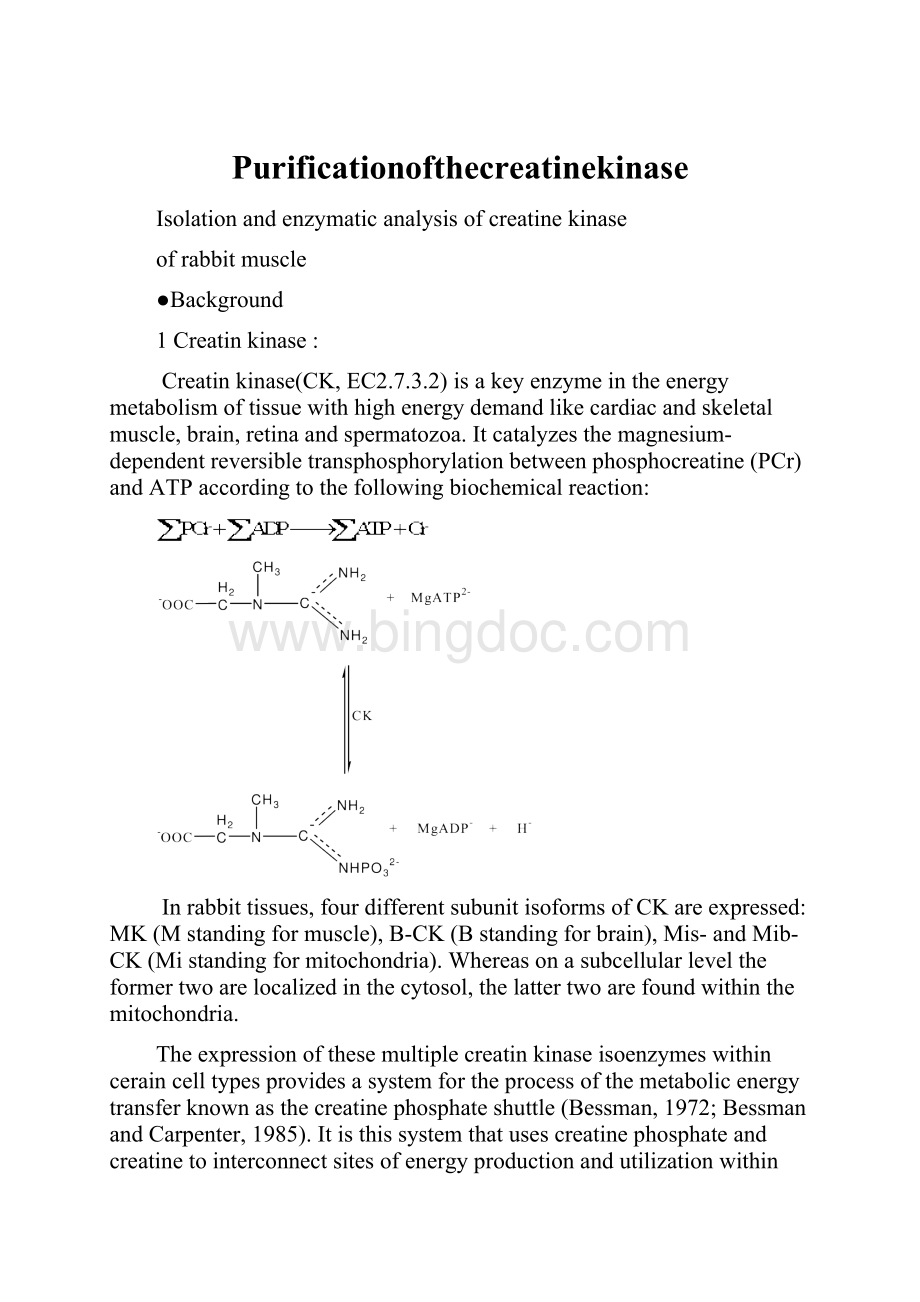
Creatinkinase(CK,EC2.7.3.2)isakeyenzymeintheenergymetabolismoftissuewithhighenergydemandlikecardiacandskeletalmuscle,brain,retinaandspermatozoa.Itcatalyzesthemagnesium-dependentreversibletransphosphorylationbetweenphosphocreatine(PCr)andATPaccordingtothefollowingbiochemicalreaction:
Inrabbittissues,fourdifferentsubunitisoformsofCKareexpressed:
MK(Mstandingformuscle),B-CK(Bstandingforbrain),Mis-andMib-CK(Mistandingformitochondria).Whereasonasubcellularleveltheformertwoarelocalizedinthecytosol,thelattertwoarefoundwithinthemitochondria.
Theexpressionofthesemultiplecreatinkinaseisoenzymeswithinceraincelltypesprovidesasystemfortheprocessofthemetabolicenergytransferknownasthecreatinephosphateshuttle(Bessman,1972;BessmanandCarpenter,1985).Itisthissystemthatusescreatinephosphateandcreatinetointerconnectsitesofenergyproductionandutilizationwithincellsfortherapidreplenishmentofmetabolicenergy.AccordingtothePCrcircuitmodel,mitochondriacreatinkinaseontheouteersurfaceoftheinnermitochondriamembranemetabolizesATP,generatedbyoxidativephosphorylationinthemitochondriamatrix,andCrtoyieldPCrandADP.PCristhoughttobetheactual“highenergycompound”diffusing,withinthecellsofthetissuesmentionedabove,toplaceswhereenergy-consumingreactionstakeplace.Atthesesites,thecytosolicCKisoenzymesregenerateATP,andtheCrtherebyproduceddiffusesbacktothemitochondria.M-CKandthetwoMi-CKsareinvolvedinthismechanism.
Besidesitsroleinenergytransfer,oneofthekeyfunctionsofcreatinkinaseinthetissuesdescribedaboveisitscapacitytomaintaincytosolicATPlevelsrelativelyconstantduringsteadystateactivityandduringmodestworktransitionsdespitedecreaseinPCr.Itperformsthisbufferfunctioninvivobymaintainingnear-equilibriumbetweenallreactioncomponets.Therexplanationare(a)theenzymebeingpresentatactivitiesintissuesfarinexcessoftherateofATPutilization,and(b)thatitisnotkineticallylimitedbyeitherofitssubstratessincetheKmvaluesareintheapproximaterangeofconcentrationreportedinthetissyue.
CKicadimericenzyme,composedoftwoidenticalsubunits.Accordingtotheprimarystructuredetermined,thenaturallyoccuringM-CKfromchicken,human,andrataswellasfromrabbitisconsistingof387aminoacidresidues,eachwithamolecularweightabout43000.Thereareeightthioylgroupsinasinglemolecule,butnodisufarbondsareformedbetweenthem.X-raydiffractionshowsthatthe3dimensionalstructureofCKmoleculeisacondensedspherewithabout25percentα-helixand15percentβ-sheet.
2Measuringtheactivityofcreatinkinase(pH--colorimetricmethod):
WhenCKcatalysestheforwardreaction,morephosphorylasesaretransferredandthesamemolecularamountofH+isgenerated.TheoptimalpHis7.5-9.0.Inthisrange,theH+generatingratecanbeusedtomeasuretheactivityofCK.Inthisexperiment,thymolblueservesaspHindicator.AspHdecreaseswithincreasingamountofgeneratingH+,thecolorofsubstratesolutionwillchangefromdarkmagentatoolivine.Whenthewavelengthis597nm,theabsorbanceA597willconstantlydecrease.
TheactivityofCKcanbecalculatedbythefollowingformula
dilutionratio(μmol/(minmg))
VA=1.0ml(Thevolumeofsubstratesolution),
VB=0.01ml(Thevolumeofenzymesolutionadded)
C:
theconcentrationoenzymesolutionadded
ΔA597:
ThechangeofA597inaminute
3Measureproteinconcentrationbyspectrophotometricassay:
Mostproteinshaverelativelyintenseultravioletlightabsorptioncenteredat280nm.Thisisduetothepresenceoftyrosineandtryptophanresiduesintheprotein.However,theamountoftheseaminoacidresiduesvariesindifferentproteins,aswaspointedoutearlier.Ifcertainprecautionsaretaken,theA280valueofproteinsolutionisproportionaltotheproteinconcentration.Theprocedureissimpleandrapid.AproteinsolutionistransferredtoaquartzcuvetteandtheA280isreadagainstareferencecuvettecontainingtheproteinsolventonly(buffer,water,etc.).
●Materialsandsupplies
1Chemicalreagents:
Form1Thechemicalreagentinvolvedinthisexperiment
Chemical
Concentration
Function
KCl
0.01mol/L
Solutetheproteinbysalt
NH4OH
1.7mol/L
ControlpH=8.0
ammoniumcitrate
0.05mol/L
Tris-HCl
0.1mol/L(pH8.0)
ControlthepH=8.0
NH4Cl
Saltoutimpurities
NH4OH
5mol/L
ControlthepH,discardtheimpurities
MgSO4
2.0mol/L(pH8.5)
SaltoutCK
MgAC2
0.07mol/L(pH9.0)
SoluteCKremaininginthepellet
NaOH
0.2,0.5,1.0,2.0,5.0mol/L
AdjustthepH
HAC
0.2mol/L
AdjustthepH
CH3CH2OH
95%
Denaturetheimpurities
Crudesalt
Maintainlowtemperature
Form2Theprotocolofsubstratesolution
Thevolumeofsubstratesolution
10ml
15ml
20ml
Creation(48mmol/L)
5ml
7.5ml
10ml
MgAC2(0.1mol/L)
0.5ml
0.75ml
1ml
thymolblue(0.1%)
1ml
1.5ml
2ml
Gly-NaOH(0.1mol/L)pH9.0
0.5ml
0.75ml
1ml
ATP
24mg
36mg
48mg
Add0.1~0.2mol/LNaOHuntilthecolorofsolutionturnspurple(A5971.8~2.0)
A5971.6~1.8
PH=9.0
A5971.6~1.8
PH=9.0
A5971.6~1.8
PH=9.0
AddH2Oto
10ml
15ml
20ml
2Instruments
(1)Specord200spectrometerforkineticspectroscopy(Germany).
(2)Speckol1200spectrometer(Germany).
(3)Constant-flowPump(China).
(4)FractionCollector(China).
(5)Bio-RADElectrophoresisSet(USA).
(6)EupaBlender(China).
●Procedure
1Preparationofcrudeextract
(1).Withasharpknife,cutthemuscleintocubes4to5cmwidefromtheice-coldrabbitmuscle.Thewhiteconnectivetissueshouldbesetaside.
(2).Quicklyweighabout50gofthemusclecubesandsuspendthecubesin150mLof0.01mol/LKClat00C.
Thethreadlikeconnectivetissueshouldbediscardedascompletelyaspossibleinthisstep.Otherwise,itmayinterferewiththestirring.
(3)Homogenize150mLofthesuspensioninaglassbeakerwithablenderfor3times(turnontheblenderathighspeedfor30secondseachtime.Theintervalbetweeneachtimeis10s)andstirthemwithaglassstirringrodat00Cfor3times.(20seachtime,withanintervalof10s).
Thestirshouldnottouchthesidewallofthebreaker.
(4)Centrifugethehomogenatefor10minutesat8000r/minat00C.Usetheelectronicbalancetobalancethetwocentrifugetube(Don'tforgetthelid)andensurethedifferencebetweentheirmassislessthan0.1g.
Ensurethatnowaterdropadheredtotheoutersidewallofthecentrifugetube.Otherwise,theadheredwatermayfreezeinthecentrifugalmachine.
(5)Carefullydecantthesupernatantandmeasurethevolumeofthesupernatant(V1)(keep0.6mLforproteinassayinthreeEPtubesat–20oC).Discardthepellet.
(Usesteelspoontodigitout)OurV1=45mL)
(6).Addgroundammoniumchloride(NH4Cl)tomake0.1mol/Lsolution
TheNH4Clweaddedis0.2354g.NH4Clshouldbeaddedslowly.Otherwiselocalconcentrationwillbesohighthattheenzymemayloseitsactivity.
Wemadeamistakeinthisstep,weaddedNH4Clrapidlyandthismayleadtotheinactivationofenzyme.
(7)AdjustpHto9.0with5mol/Lammoniumhydroxide(NH4OH),keepstirringthesolutionat00Cfor30minutes.
TheNH4OHshouldbeaddedhalfadroponetimeandmeasurethepHeachtime.
(8)AddV1ofcold95%ethanolandstirthesolutionat200Cfor30minutes.
(9)Centrifugethesolutionfor10minutesat8000r/minat-80C.Discardthepelletandmeasurethevolumeofthesupernatant(V2)(keep0.6mLforproteinassayat–200C).
V2=68mL
(10)Takethesupernatant(V2)andaddVA(mL)of2mol/LMgSO4(pH8.5)toafinalconcentrationof0.03mol/L.
VA=1.020mL
(10)AddVAofcold95%ethanolandstirthesolutionat00Cfor30minutes.
(11)Centrifugethesolutionfor10minutesat8000r/minat-80C.Pouroffanddiscardthesupernatantandassemblethepelletinatube.
Inprinciple,thesolutionshouldbedividedin4centrifugetubes,butouramountisrelativelylittle,todecreasetheloss,wedividedthesolutionin3tubesandfillwaterintheforthtubetobalance.
(12).Suspendthepelletwith1/10V10.07mol/LMgAC2(pH9.0).
Actually,wedidn'tdothisstepinthefirstexperiment.
(13)Storethepelletat–200Covernight.
(14).SolvethepelletinMgAc2.Stirthesuspensionat0oCfor30minutes.Centrifugethesuspensionfor10minutesat12000r/minat00C.Pouroffanddiscardthepellet.
ThevolumeofMgAc2=1/10thevolumeofV1,soweadded4.5mLMgAc2(aq)
(15).Measurethevolumeofthesupernatant(V3).Keep0.6mLforproteinassayinEPtubesat–200C(0.2mLineachtube).
OurV3=6.4mL
(16)AdjustthepHofthesupernatantto8.0with0.5mol/LNaOHandaddVBofcold95%ethanoltoafinalconcentrationof36%inanice-saltbath.CalculatetheVBusing:
(V3-thevolumeofMgAC2)x0.5+VB/(V3+VB)=0.36
OurVB=2.1156mL
Beforeadding,wepreparedtheicesaltbath.Whatisimportantisthatthesaltshouldbedistributearoundthebreakerbutavoiddistributingitintothebreaker.
Otherwise,theenzymewillbesaltoutorinactivated.
Unfortunately,inthisstep,wedidn'tgetanysediment.Perhaps,ourenzymeispureenoughormaybewedidn'tsetthesysteminaconditionthatfavorstheformationofproteinsediment.
(17)Stirthesolutionat00Cfor30minutes.Centrifugefor10minutesat12000r/minat
-80C.Pouroffanddiscardthepelletandkeep0.6mLforproteinassayin3EPtubesat–200C(02mLineachtube).Measurethevolumeofthesupernatant(V4).